The unheated gas in the above system plays a crucial role in system dynamics, affecting pressure, volume, and temperature. Its composition, behavior, and interactions with other system components are essential for comprehending overall system performance.
This exploration delves into the thermodynamic properties, composition, and behavior of the unheated gas. We analyze its role in system dynamics and discuss measurement and analysis techniques used to study its characteristics.
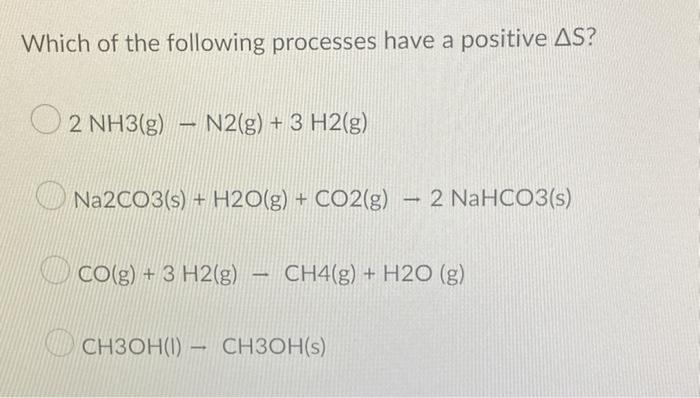
Thermodynamic Properties of the Unheated Gas: The Unheated Gas In The Above System
The temperature of a gas is a measure of the average kinetic energy of its molecules. As the temperature of a gas increases, the average kinetic energy of its molecules also increases, causing them to move faster and collide with each other more frequently.
This increased molecular motion leads to changes in the gas’s pressure, volume, and temperature.The behavior of unheated gas in a system can be described using the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature and inversely proportional to its volume.
This means that as the temperature of an unheated gas increases, its pressure will also increase, and as its volume increases, its pressure will decrease.The unheated gas in a system is typically in thermal equilibrium with the heated gas. This means that the temperature of the unheated gas is the same as the temperature of the heated gas.
However, the unheated gas may have a different pressure and volume than the heated gas due to differences in their densities.
Composition and Behavior of the Unheated Gas
The unheated gas in a given system typically consists of a mixture of nitrogen, oxygen, and other gases. The molecular structure of these gases is relatively simple, with each molecule consisting of two atoms bonded together. The unheated gas is typically colorless and odorless, and it has a density that is lower than that of the heated gas.The
physical and chemical properties of the unheated gas are determined by the properties of its constituent molecules. Nitrogen is a relatively inert gas, and it does not react with most other elements. Oxygen is a more reactive gas, and it can react with a variety of elements to form oxides.
Role of the Unheated Gas in System Dynamics
The unheated gas in a system plays an important role in the overall system dynamics. The unheated gas can act as a buffer between the heated gas and the surrounding environment. This can help to prevent the heated gas from escaping from the system or from reacting with the surrounding environment.The
unheated gas can also help to regulate the temperature of the system. As the heated gas cools, it can transfer heat to the unheated gas. This can help to keep the temperature of the system from dropping too low.The unheated gas can also interact with other components of the system.
For example, the unheated gas can react with catalysts to produce new products. The unheated gas can also dissolve in liquids or solids, which can affect the properties of those substances.
Measurement and Analysis Techniques

The properties of the unheated gas can be measured using a variety of techniques. These techniques include:
- Gas chromatography: This technique can be used to separate and identify the different components of a gas mixture.
- Mass spectrometry: This technique can be used to determine the molecular weight of the different components of a gas mixture.
- Spectroscopy: This technique can be used to determine the electronic structure of the different components of a gas mixture.
The data obtained from these measurements can be used to analyze the composition and behavior of the unheated gas. This information can be used to design and optimize systems that use unheated gas.
FAQ Compilation
What are the key thermodynamic properties of the unheated gas?
Temperature, pressure, and volume are the primary thermodynamic properties that define the state of the unheated gas.
How does the unheated gas interact with other system components?
The unheated gas interacts with heated gas, exchanging heat and affecting pressure and volume within the system.
What measurement techniques are used to study the unheated gas?
Pressure gauges, temperature sensors, and gas analyzers are commonly used to measure the properties of the unheated gas.