Section 15.1 the composition of seawater – Section 15.1: The Composition of Seawater embarks on an enlightening journey, unraveling the intricate tapestry of the ocean’s chemical composition. This detailed exploration delves into the major components, ions, and minor constituents that define the life-sustaining properties of seawater.
From the vast expanse of the open ocean to the depths of the abyss, the composition of seawater plays a pivotal role in shaping marine ecosystems and supporting the diverse array of organisms that call it home.
Components of Seawater: Section 15.1 The Composition Of Seawater
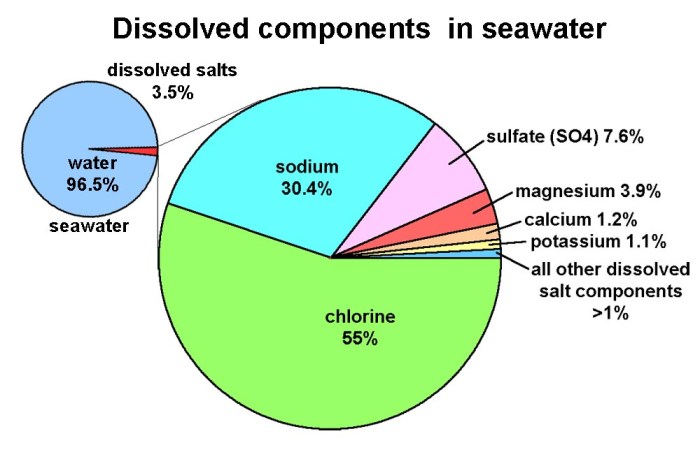
Seawater is a complex mixture of various dissolved substances. The major components of seawater include:
- Water (H2O): The primary constituent of seawater, accounting for approximately 96.5% of its mass.
- Sodium chloride (NaCl):The most abundant salt in seawater, contributing about 86% of the total dissolved solids.
- Magnesium chloride (MgCl2): The second most abundant salt, accounting for approximately 11% of the dissolved solids.
- Calcium sulfate (CaSO4): The third most abundant salt, constituting about 3% of the dissolved solids.
- Potassium chloride (KCl):A minor salt, but still significant, making up about 2% of the dissolved solids.
In addition to these major salts, seawater also contains dissolved gases, such as:
- Oxygen (O2): Essential for marine life, oxygen is dissolved in seawater from the atmosphere and through photosynthesis.
- Carbon dioxide (CO2): Dissolved in seawater from the atmosphere and through biological processes.
- Nitrogen (N2): The most abundant gas in seawater, dissolved from the atmosphere.
The salinity of seawater varies with depth and location. In general, salinity increases with depth due to the accumulation of dissolved salts. Salinity also varies geographically, influenced by factors such as evaporation, precipitation, and freshwater inputs from rivers and glaciers.
Major Ions in Seawater
| Ion | Concentration (mg/L) | Charge |
|---|---|---|
| Sodium (Na+) | 10,800 | +1 |
| Chloride (Cl–) | 19,400 | -1 |
| Magnesium (Mg2+) | 1,350 | +2 |
| Calcium (Ca2+) | 410 | +2 |
| Potassium (K+) | 390 | +1 |
| Sulfate (SO42-) | 2,700 | -2 |
| Bicarbonate (HCO3–) | 140 | -1 |
| Bromide (Br–) | 65 | -1 |
The major ions in seawater originate from various sources, including volcanic eruptions, weathering of rocks, and biological processes. These ions play crucial roles in marine ecosystems, participating in physiological processes, maintaining osmotic balance, and supporting the growth of marine organisms.
The concept of ionic balance in seawater refers to the balance between the positive and negative charges of the major ions. This balance is maintained through the presence of equal amounts of positive and negative charges, ensuring the electrical neutrality of seawater.
Minor Constituents of Seawater
- Trace elements:Essential for marine life, trace elements include iron, copper, zinc, and manganese.
- Organic matter:Derived from living and decaying organisms, organic matter contributes to the carbon cycle and supports marine food webs.
- Suspended particles:These include sediment, plankton, and other small particles that can affect water clarity and light penetration.
- Human-made pollutants:Unfortunately, human activities can introduce pollutants into seawater, such as plastics, pesticides, and heavy metals.
Trace elements are vital for marine life, participating in enzymatic reactions, hormone production, and other physiological processes. Organic matter provides a food source for marine organisms and contributes to the cycling of nutrients. Suspended particles can influence the behavior of marine animals, such as filter feeders, and affect the availability of light for photosynthesis.
Human-made pollutants can have detrimental effects on marine ecosystems, harming organisms and disrupting ecological processes. It is crucial to minimize the release of pollutants into seawater to protect marine environments.
pH and Alkalinity of Seawater, Section 15.1 the composition of seawater
pH is a measure of the acidity or alkalinity of a solution. The pH of seawater typically ranges from 7.8 to 8.2, indicating a slightly alkaline nature.
Alkalinity refers to the capacity of seawater to neutralize acids. It is primarily influenced by the presence of carbonate and bicarbonate ions, which act as buffers. The alkalinity of seawater is important for maintaining a stable pH and supporting the growth of marine organisms that utilize calcium carbonate for shell formation.
Factors that can influence the pH and alkalinity of seawater include:
- Temperature:Warmer water has a lower pH than colder water.
- Salinity:Higher salinity generally results in higher pH.
- Biological activity:Photosynthesis by marine plants consumes carbon dioxide, increasing the pH.
Changes in pH and alkalinity can have significant implications for marine ecosystems. For example, ocean acidification, caused by the absorption of atmospheric carbon dioxide, can reduce the availability of carbonate ions and make it more difficult for marine organisms to build shells.
Key Questions Answered
What are the major components of seawater?
The major components of seawater include sodium chloride (NaCl), magnesium chloride (MgCl2), calcium sulfate (CaSO4), and potassium chloride (KCl).
How does salinity vary with depth and location?
Salinity generally increases with depth and varies with location due to factors such as evaporation, precipitation, and freshwater input from rivers and glaciers.
What is the role of trace elements in marine life?
Trace elements are essential for the growth and survival of marine organisms, playing vital roles in metabolic processes, enzyme function, and skeletal development.

