Which of the following processes have a δs 0 – Which of the following processes have a δS = 0? This question delves into the realm of thermodynamics, where the interplay of energy, entropy, and spontaneity unfolds. As we embark on this journey, we will uncover the fundamental principles that govern the direction and spontaneity of various processes.
Gibbs free energy, entropy, enthalpy, and coupled reactions, each play a pivotal role in determining whether a process is spontaneous or not. By exploring these concepts, we will gain a deeper understanding of the factors that influence the behavior of chemical systems.
Thermodynamics and Gibbs Free Energy

Gibbs free energy (G) is a thermodynamic potential that measures the maximum amount of work that can be extracted from a thermodynamic system at constant temperature and pressure. The change in Gibbs free energy (δG) for a process is given by the equation:
δG = δH – TδS
where δH is the change in enthalpy, T is the absolute temperature, and δS is the change in entropy.
Processes with δG > 0, Which of the following processes have a δs 0
Processes with a positive change in Gibbs free energy (δG > 0) are nonspontaneous. This means that they will not occur spontaneously without the input of external energy.
- The dissolution of a solid in a solvent.
- The condensation of a gas.
- The freezing of a liquid.
Processes with δG < 0
Processes with a negative change in Gibbs free energy (δG< 0) are spontaneous. This means that they will occur spontaneously without the input of external energy.
- The reaction of an acid and a base.
- The combustion of a fuel.
- The flow of heat from a hot object to a cold object.
Entropy and the Second Law of Thermodynamics

Entropy (S) is a measure of the disorder or randomness of a system. The Second Law of Thermodynamics states that the total entropy of an isolated system always increases over time.
The change in entropy of a system (δS) is given by the equation:
δS = Q/T
where Q is the heat added to the system and T is the absolute temperature.
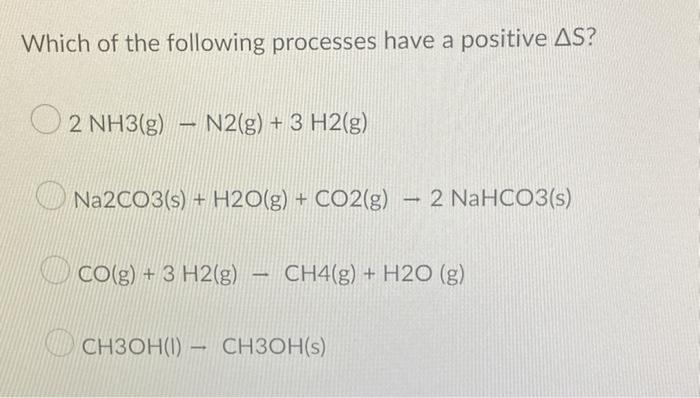
Processes with δS > 0
Processes with a positive change in entropy (δS > 0) are spontaneous. This means that they will occur spontaneously without the input of external energy.
- The mixing of two gases.
- The expansion of a gas into a vacuum.
- The melting of a solid.
Enthalpy and Temperature
Enthalpy (H) is a thermodynamic potential that measures the total energy of a system. The change in enthalpy (δH) for a process is given by the equation:
δH = Q – W
where Q is the heat added to the system and W is the work done by the system.
The relationship between temperature and enthalpy change is given by the equation:
δH = C pδT
where C pis the heat capacity of the system at constant pressure and δT is the change in temperature.
Processes with δH > 0
Processes with a positive change in enthalpy (δH > 0) are nonspontaneous. This means that they will not occur spontaneously without the input of external energy.
- The combustion of a fuel.
- The freezing of a liquid.
- The condensation of a gas.
Coupled Reactions and Le Chatelier’s Principle: Which Of The Following Processes Have A δs 0
Coupled reactions are two or more reactions that occur simultaneously. The overall Gibbs free energy change (δG) for a coupled reaction is the sum of the Gibbs free energy changes for the individual reactions.
Le Chatelier’s Principle states that if a stress is applied to a system at equilibrium, the system will shift in a direction that relieves the stress.
Coupled Reactions with δG < 0
Coupled reactions with a negative overall Gibbs free energy change (δG< 0) are spontaneous. This means that they will occur spontaneously without the input of external energy.
One example of a coupled reaction with a negative overall Gibbs free energy change is the Haber process, which is used to produce ammonia.
N 2(g) + 3H 2(g) → 2NH 3(g)
The Gibbs free energy change for this reaction is negative, so it is spontaneous. However, the reaction is slow at room temperature. To increase the rate of the reaction, it is necessary to apply a stress to the system, such as increasing the pressure or temperature.
Essential Questionnaire
What is Gibbs free energy?
Gibbs free energy is a thermodynamic potential that measures the maximum amount of work that can be extracted from a thermodynamic system at constant temperature and pressure.
What is entropy?
Entropy is a measure of the disorder or randomness of a system.
What is enthalpy?
Enthalpy is a thermodynamic quantity equivalent to the total thermal energy of a system.
