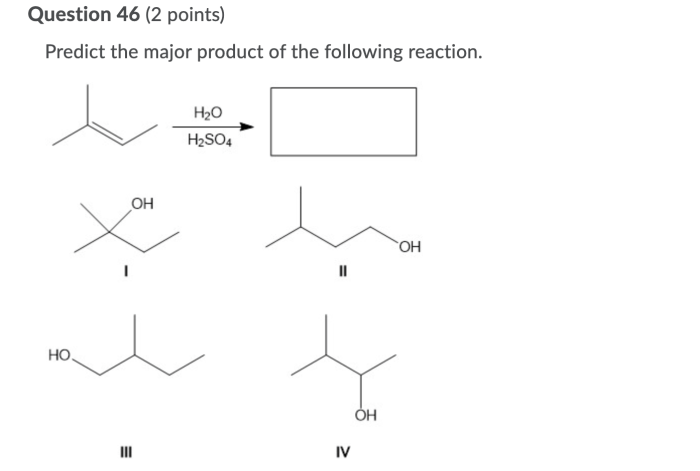
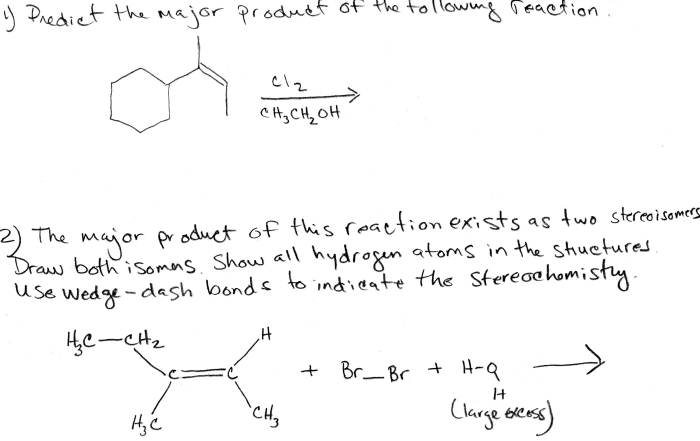
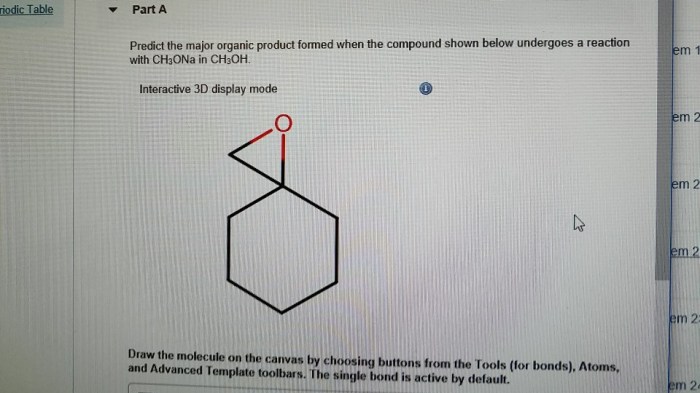
Predict the major product for the reaction shown – Predicting the major product for a given reaction is a fundamental skill in organic chemistry. It requires a deep understanding of reaction mechanisms, regioselectivity, stereoselectivity, and other factors that influence the formation of products. This guide provides a comprehensive overview of these concepts and their application in predicting major products.
The ability to predict major products is essential for designing synthetic strategies, understanding reaction pathways, and optimizing reaction conditions. This guide will equip readers with the knowledge and tools necessary to master this critical aspect of organic chemistry.
Predicting Major Products in Organic Reactions
Predicting the major product in an organic reaction is a crucial skill for organic chemists. It allows us to design synthetic strategies and understand the reactivity of organic compounds. The formation of a major product is influenced by several factors, including the reaction mechanism, regioselectivity, stereoselectivity, and other factors such as temperature, solvent effects, and catalyst effects.
Reaction Mechanism
The reaction mechanism is the step-by-step process by which a reaction occurs. Understanding the reaction mechanism is essential for predicting the major product because it allows us to identify the key steps involved in the formation of the major product.
For example, in the SN2 reaction, the nucleophile attacks the electrophile at the backside, leading to the formation of an inverted product. This is because the nucleophile attacks the electrophile from the opposite side of the leaving group.
Regioselectivity
Regioselectivity refers to the preference for one reaction site over another. In many reactions, there are multiple possible reaction sites, and the major product is the one that is formed by the reaction at the most reactive site. For example, in the addition of HCl to an alkene, the major product is the Markovnikov product, which is formed by the addition of HCl to the more substituted carbon of the double bond.
Stereoselectivity
Stereoselectivity refers to the preference for one stereoisomer over another. In many reactions, there are multiple possible stereoisomers, and the major product is the one that is formed by the reaction that leads to the most stable stereoisomer. For example, in the addition of HBr to an alkene, the major product is the anti-Markovnikov product, which is formed by the addition of HBr to the less substituted carbon of the double bond.
Other Factors, Predict the major product for the reaction shown
In addition to the reaction mechanism, regioselectivity, and stereoselectivity, other factors can influence the formation of major products. These factors include temperature, solvent effects, and catalyst effects. For example, the temperature of a reaction can affect the rate of the reaction and the selectivity of the reaction.
The solvent can also affect the selectivity of the reaction by stabilizing certain transition states. Catalysts can also affect the selectivity of the reaction by providing an alternative pathway for the reaction to occur.
Examples
The following table shows the major products of different reactions. The reactions are listed in order of increasing complexity.
| Reaction | Major Product |
|---|---|
| SN2 reaction | Inverted product |
| Addition of HCl to an alkene | Markovnikov product |
| Addition of HBr to an alkene | Anti-Markovnikov product |
| Diels-Alder reaction | Endo product |
| Claisen rearrangement | Ortho product |
FAQ: Predict The Major Product For The Reaction Shown
What are the key factors that influence the formation of major products in organic reactions?
The key factors include the stability of the transition state, the strength of the nucleophile and electrophile, steric effects, electronic effects, and solvent effects.
How can regioselectivity be used to predict major products?
Regioselectivity refers to the preference for one regioisomer over others. It can be predicted based on factors such as the stability of the carbocation intermediate, the Markovnikov’s rule, and the orientation of the double bond.
What is the difference between regioselectivity and stereoselectivity?
Regioselectivity refers to the preference for one regioisomer, while stereoselectivity refers to the preference for one stereoisomer. Regioselectivity is determined by the position of the functional group, while stereoselectivity is determined by the orientation of the atoms in space.