Balancing chemical equations gizmo answer key activity b is a valuable tool for students seeking to enhance their understanding of chemical reactions. This comprehensive guide will provide an in-depth exploration of the Balancing Chemical Equations Gizmo and its applications, offering a step-by-step approach to balancing equations and a detailed analysis of the combustion reaction experiment in Activity B.
This guide delves into the significance of balancing chemical equations, explaining how it ensures that the number of atoms of each element on the reactants’ side of an equation matches the number of atoms of that element on the products’ side.
The Balancing Chemical Equations Gizmo, an interactive simulation, is introduced as a valuable tool for visualizing and balancing equations, making the process more accessible and engaging.
Balancing Chemical Equations: Balancing Chemical Equations Gizmo Answer Key Activity B
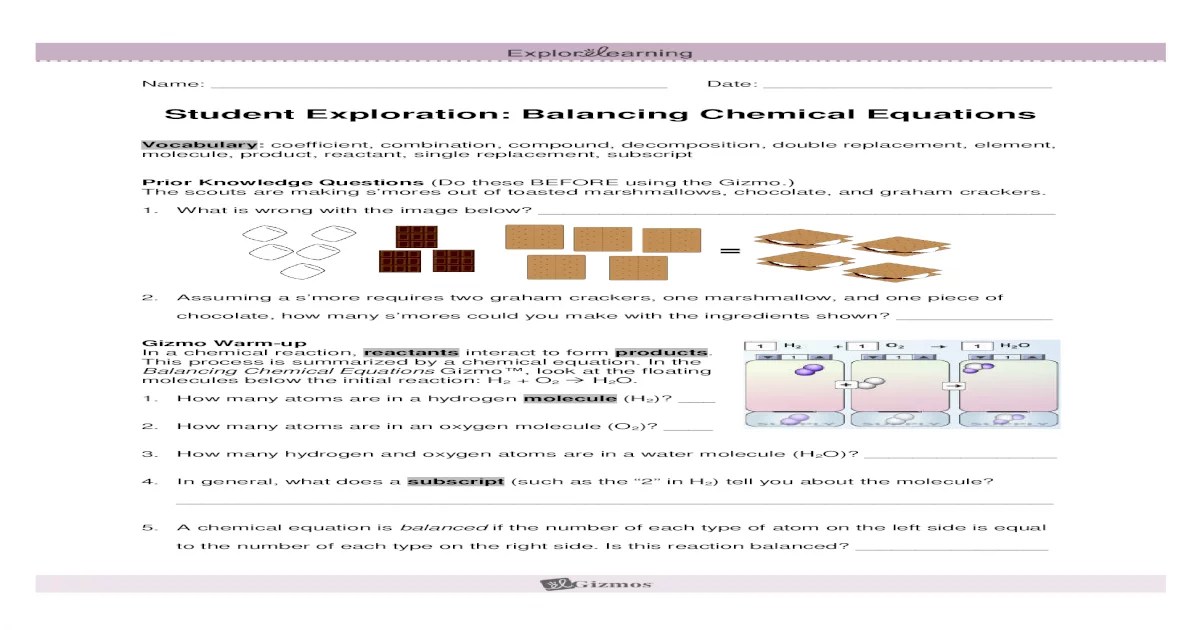
Balancing chemical equations is essential for understanding chemical reactions and predicting the products and reactants involved. A balanced chemical equation ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side.
The Balancing Chemical Equations Gizmo is a virtual laboratory tool that allows students to explore the process of balancing chemical equations. Activity B of the Gizmo focuses on combustion reactions, which are chemical reactions that involve the burning of a fuel with oxygen.
Balancing Equations
To balance a chemical equation, follow these steps:
- Write the unbalanced equation.
- Identify the element that is not balanced.
- Add coefficients to the reactants or products to balance the number of atoms of that element.
- Repeat steps 2 and 3 until all elements are balanced.
Coefficients represent the number of molecules or moles of a reactant or product. They must be used carefully to ensure that the equation remains balanced.
The Balancing Chemical Equations Gizmo can be used to balance equations by entering the unbalanced equation into the Gizmo and clicking the “Balance” button. The Gizmo will automatically adjust the coefficients to balance the equation.
Activity B: Combustion Reactions, Balancing chemical equations gizmo answer key activity b
Activity B of the Balancing Chemical Equations Gizmo involves the following steps:
- Select a fuel from the dropdown menu.
- Adjust the amount of fuel and oxygen used in the reaction.
- Click the “React” button to start the reaction.
- Observe the products of the reaction.
The Gizmo allows students to explore the effects of changing the amount of fuel and oxygen on the products of the reaction. Students can also use the Gizmo to determine the balanced chemical equation for the reaction.
Applications
Balancing chemical equations has numerous applications, including:
- Predicting the products and reactants of chemical reactions
- Determining the stoichiometry of reactions
- Calculating the amount of reactants or products needed for a given reaction
Balancing chemical equations is an essential skill for anyone who wants to understand chemistry. The Balancing Chemical Equations Gizmo is a valuable tool that can help students learn this important skill.
Essential FAQs
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of an equation matches the number of atoms of that element on the products’ side, reflecting the conservation of mass in chemical reactions.
How can I use the Balancing Chemical Equations Gizmo to balance equations?
The Balancing Chemical Equations Gizmo provides an interactive simulation where users can input unbalanced equations and the Gizmo will automatically balance them. It allows for visual representation of the balancing process, making it more intuitive and engaging.
What is the combustion reaction experiment in Activity B?
Activity B involves a combustion reaction experiment where students burn different fuels and measure the amount of heat released. This experiment demonstrates the principles of combustion reactions and provides data for analyzing the efficiency of different fuels.